Point-of-care Testing Program
- Home
- Practice, Teaching & E...
- Public & Professional ...
- Point-of-care Testing ...

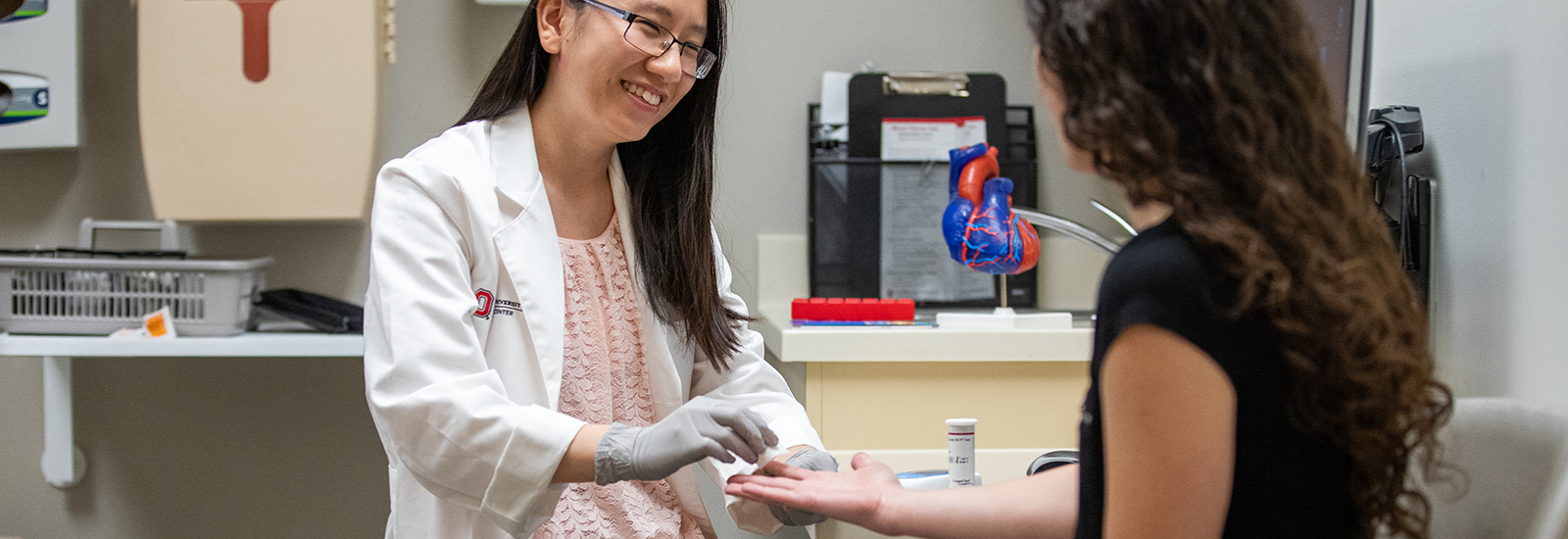
The Ohio State University College of Pharmacy Point-of-care Testing (POCT) Program is designed to certify and train our students to conduct CLIA-waived testing in community settings. For now, this is limited to blood glucose screenings, although we are always looking to expand our program where appropriate.
What is the POCT Program?
The POCT program is a voluntary certification program at the College of Pharmacy that allows students, once they complete training and earn their certification, to participate in CLIA-waived laboratory tests, specifically blood glucose screenings, under the direct supervision of a preceptor.
Student Pharmacist POCT Certification
In order to conduct testing, each student is required to complete the college's Point-of-care Testing Program training. This certification process is voluntary but is required in order to conduct testing under the college’s CLIA waiver. This program consists of several online modules in Carmen Canvas and one in-person training. The online modules are required to be completed prior to the in-person testing.
- Students must provide proof of certification to the event coordinator at each event
- Certificates are valid until October 1 of the following academic year
- Students must be recertified each year
About the CLIA Waiver
- What is “CLIA”?
“CLIA” is the acronym for the Clinical Laboratory Improvement Amendments of 1988. This law requires any facility performing examinations of human specimens (e.g., tissue, blood, urine, etc.) for diagnosis, prevention, or treatment purposes to be certified by the Secretary of the Department of Health and Human Services. - Why is CLIA important?
For many Americans, the accuracy of clinical laboratory test results can be a life-or-death matter. If glucose tests are not performed correctly, a patient could receive an incorrect insulin dose and sustain potentially dangerous consequences. If your cholesterol is high and the laboratory results are reported as normal, you may not receive the care necessary to prevent a heart attack. - What is waived testing?
By the CLIA law, waived tests are those tests that are determined by CDC or FDA to be simple enough that there is little risk of error as long as you follow manufacturer instructions. Some testing methods for glucose and cholesterol are waived along with pregnancy tests, fecal occult blood tests, some urine tests, etc. Currently, 40 tests have been approved for certificate of waiver (COW) status at CLIA website http://www.fda.gov/cdrh/clia. There is a difference between CLIA waived tests for professional and public use.
Accessing the Training
Every PharmD student is enrolled at the beginning of the year in the current year’s POCT training. If for some reason you have trouble accessing the course content, please reach out to the contacts below.
Upcoming Opportunities
Check with your student organization or the POCT course in Carmen for opportunities thorughout the year.
Partner with us!
Our community events are successful because of many partnerships with our local communities and pharmacists. Please reach out to us if you would like to partner to host a screening opportunity or if you are interested in precepting our students at one of these events.
To learn more, contact Emily Keeler at Keeler.16@osu.edu or Dr. Jen Rodis at Rodis.2@osu.edu.